Disease background
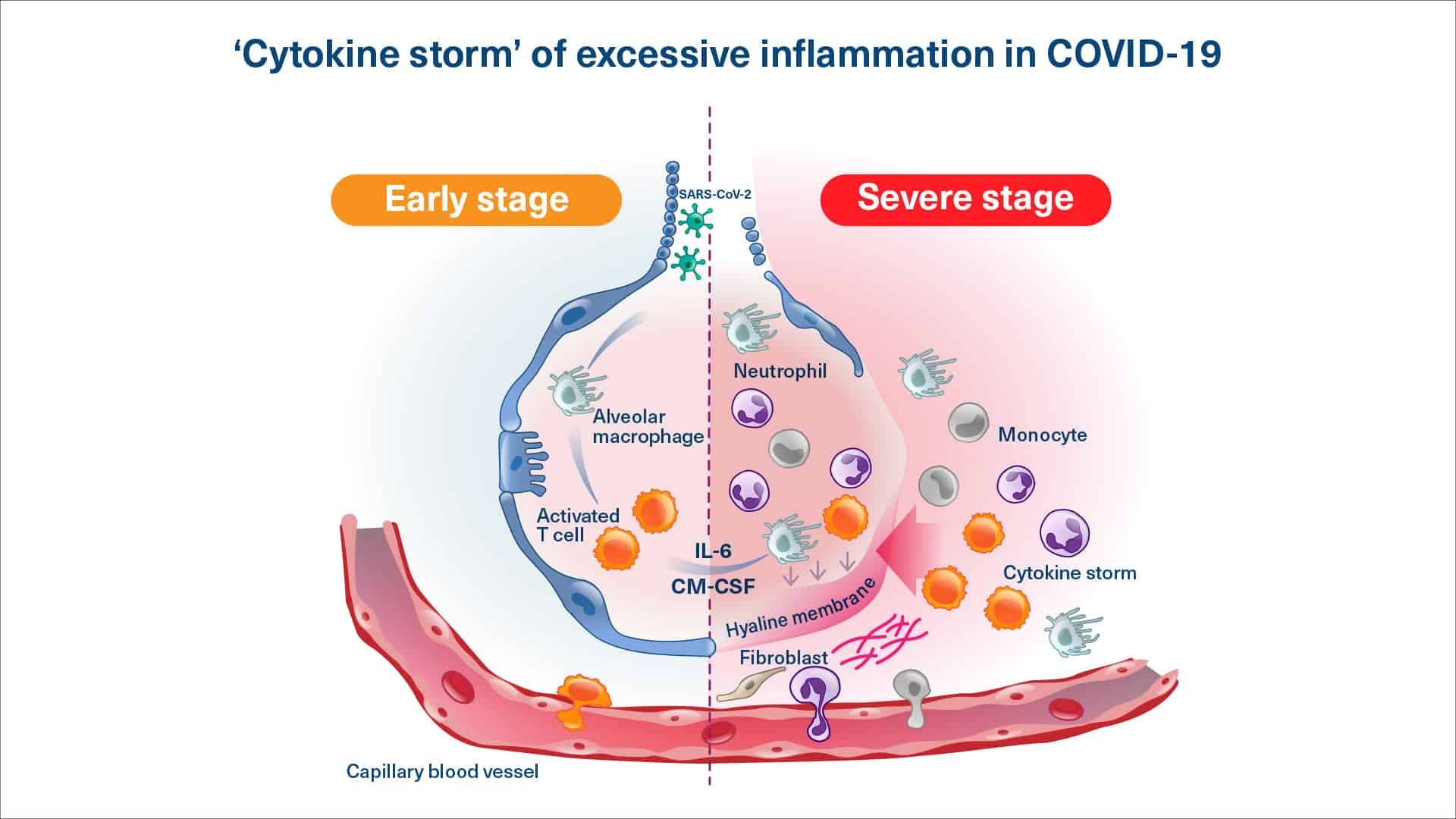
Virus infected patients can develop a variety of symptoms, but lung involvement is very common and in some viral infections like COVID-19 it can be the leading cause of death. Patients can develop respiratory insufficiency where they are unable to provide enough oxygen to the body and these patients require oxygen supplementation to maintain adequate levels. As respiratory insufficiency continues it can cause severe pneumonia and can also develop into acute respiratory distress syndrome (ARDS), a very serious condition where patients often require mechanical ventilation in order to breathe adequately.
Viral or secondary bacterial infections can also cause the immune system to be highly overly active and produce excessive quantities of pro-inflammatory molecules (a ‘cytokine storm’, also known as Systemic Inflammatory Distress Syndrome or SIDS) which can cause damage to key organ systems like the lungs, kidneys, and heart.
Viral infections can cause significant respiratory issues. To prevent the inflammation-associated damage that viral infections can cause, it is important to resolve the excessive inflammation without suppressing the immune system’s ability to fight the viral infection. The goal of therapy would be to arrest the excessive inflammation and prevent severe disease.
RESOVIR
Resomelagon (AP1189) has been tested in animal models with SIDS and shown to selectively reduce inflammation and stimulate macrophages to clean-up the inflamed tissue. In order to effectively investigate the potential for resomelagon (AP1189) to benefit viral infected patients, we formed the RESOVIR (Resolution Therapy for Viral Inflammation Research) collaboration. RESOVIR is a scientific and clinical collaboration between Professor Mauro Teixeira, MD, PhD, Universidade Federal de Minas, Belo Horizonte, Brazil, Professor Mauro Perretti, PhD William Heavy Research Institute, Barts and the London School of Medicine, Queen Mary University, London, UK, and SynAct Pharma AB. The aim of the RESOVIR collaboration is to investigate the utility of resolution therapy to resolve the cytokine storm inflammation associated with significant viral infections.
Clinical development of resomelagon (AP1189) in COVID-19-induced respiratory insufficiency
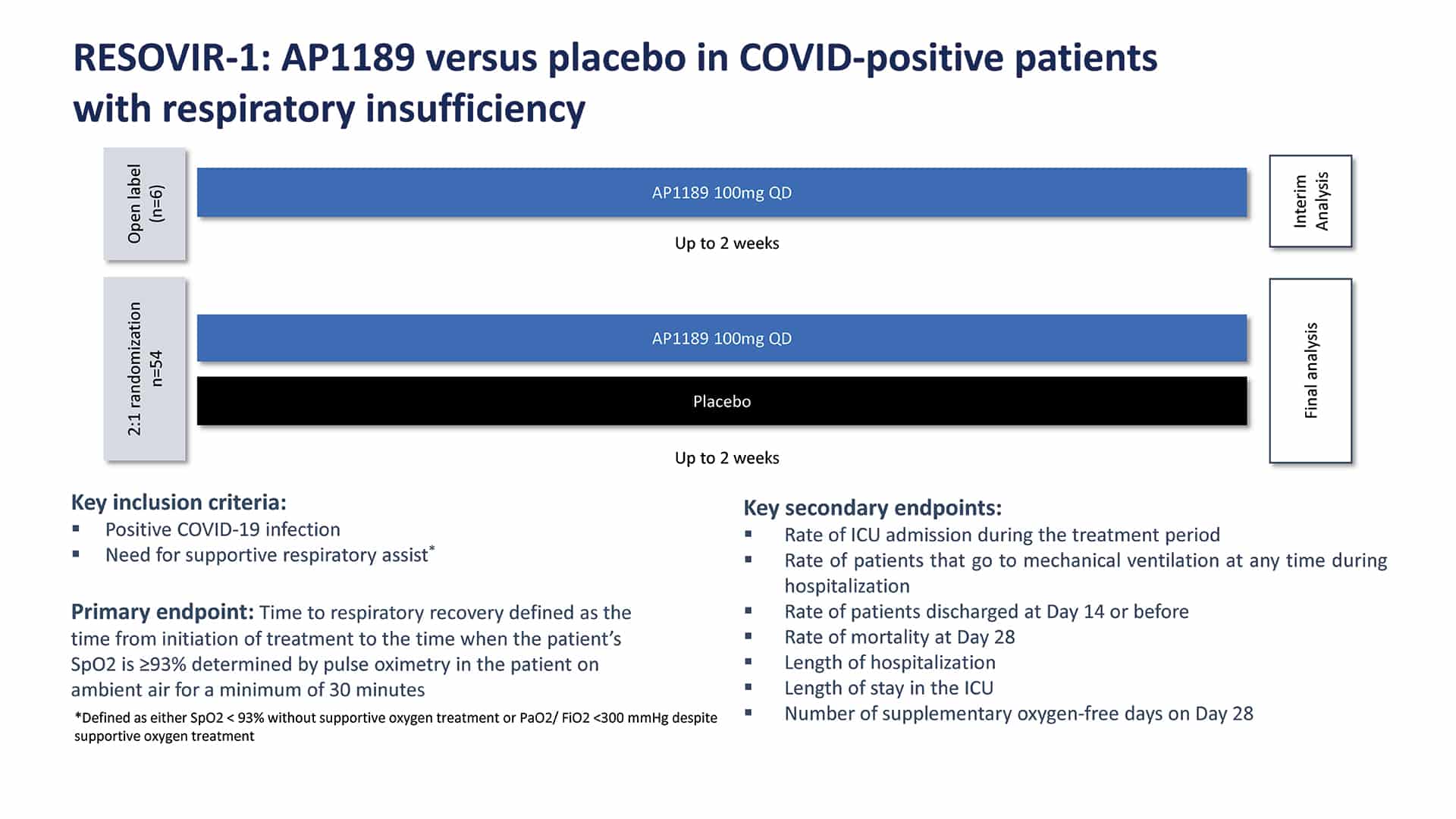
Working within the RESOVIR collaboration, we designed and executed a 60 patient Phase 2a clinical trial in Brazil. Hospitalized COVID-19 infected patients were enrolled in the study who required supplemental oxygen (experiencing respiratory insufficiency). These patients were hospitalized, and all received steroids (dexamethasone) at an average dose of 6mg/day. After an initial open-label safety run-in of 6 patients, the blinded placebo-controlled portion of the trial dosed an additional 36 patients with 100mg of resomelagon (AP1189) and 18 patients with placebo, each given orally once-daily for 2 weeks.
Results
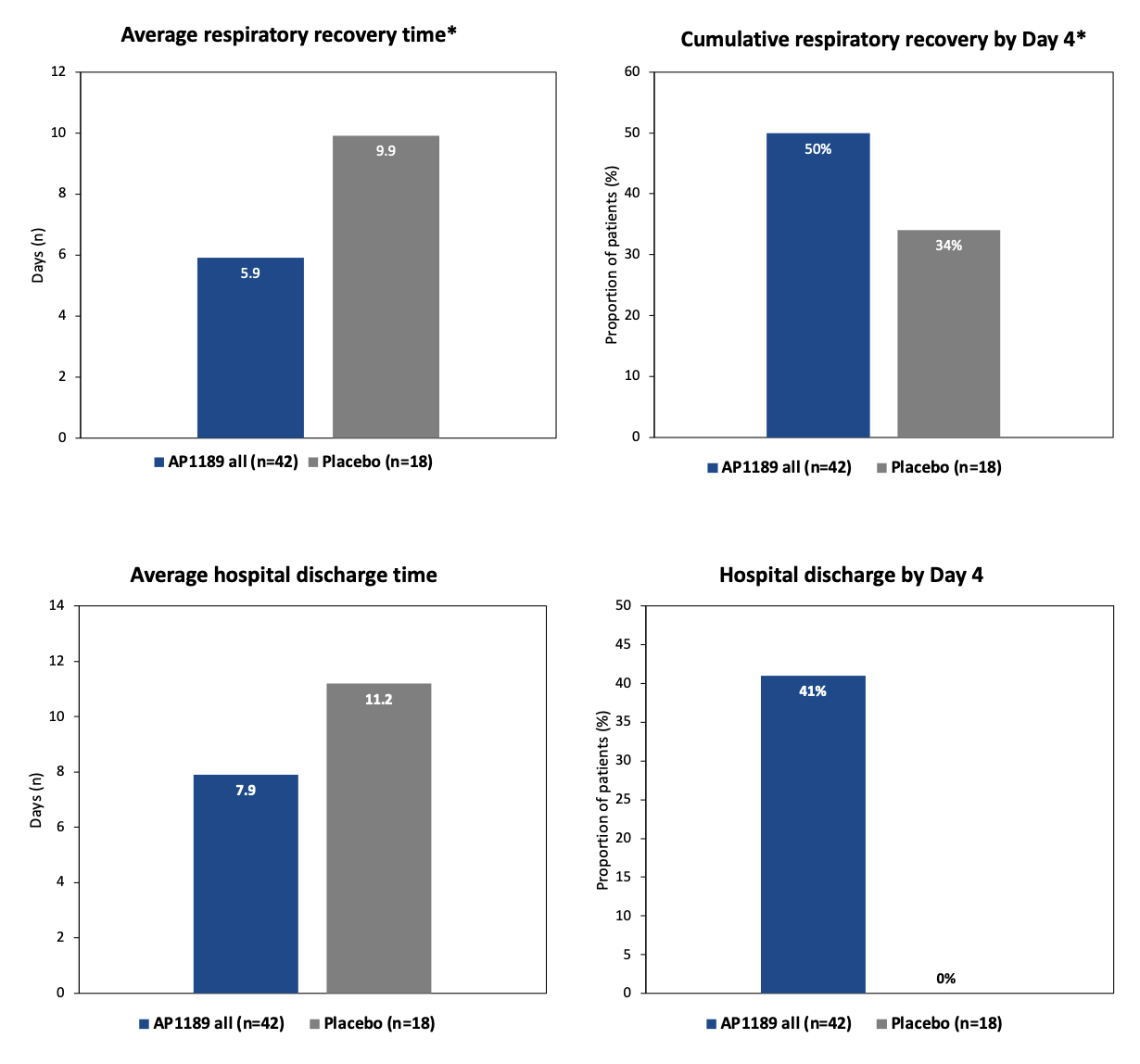
The trial concluded in Q2 of 2021. Patients treated with 100mg resomelagon (AP1189) orally once-daily for 2-weeks achieved respiratory recovery (no longer requiring oxygen therapy) on average 3.5 days (35%) quicker than placebo treated patients (6.4 days and 9.9 days on average respectively). All resomelagon (AP1189) treated patients (including the first 6 open-label safety patients) recovered respiratory recovery on average 4.0 days (40%) quicker than placebo treated patients (5.9 days and 9.9 days on average respectively). Resomelagon (AP1189) patients were discharged on average 3.3 days earlier than placebo and by day 4, 41% of resomelagon (AP1189) patients had been discharged vs 0% for placebo.
Next Steps for resomelagon (AP1189) in virus-induced respiratory insufficiency
SynAct has explored various opportunities for further development of resomelagon (AP1189) for use in patients suffering from COVID-19. Omicron and subsequent COVID-19 variants appear to induce a lower incidence of severe disease resulting in fewer hospitalized patients and the indication is therefore not pursued.
Virus-induced respiratory insufficiency is also associated with common annual or seasonal viral infections such as viral pneumonia and or influenza. Thus, SynAct has initiated pre-clinical pharmacological studies in virus models with the aim of informing decisions on next steps for the program including the design of a potential next clinical study.