Disease Background
Rheumatoid arthritis (RA) is a chronic inflammatory disorder that typically affects more than just the joints. RA is an autoimmune disorder, a disease where the immune system mistakenly attacks your body’s own tissues. RA affects the lining of the joints, causing a painful swelling that can result in cartilage and bone erosion and joint deformity. RA is often associated with symptoms involving other parts of the body including the skin, eyes, lungs, heart, and blood vessels. While new types of medications have improved treatment options, significant unmet needs still exist. For most patients, RA still progresses, and damage accumulates. Patients cycle through therapies and classes of therapies and must deal with periods of acute disease activity called flares, which can occur several times per year and drive the need to adjust the dose of current drugs or to change to a new therapy to maintain control of the disease.
Clinical development of resomelagon (AP1189) in RA
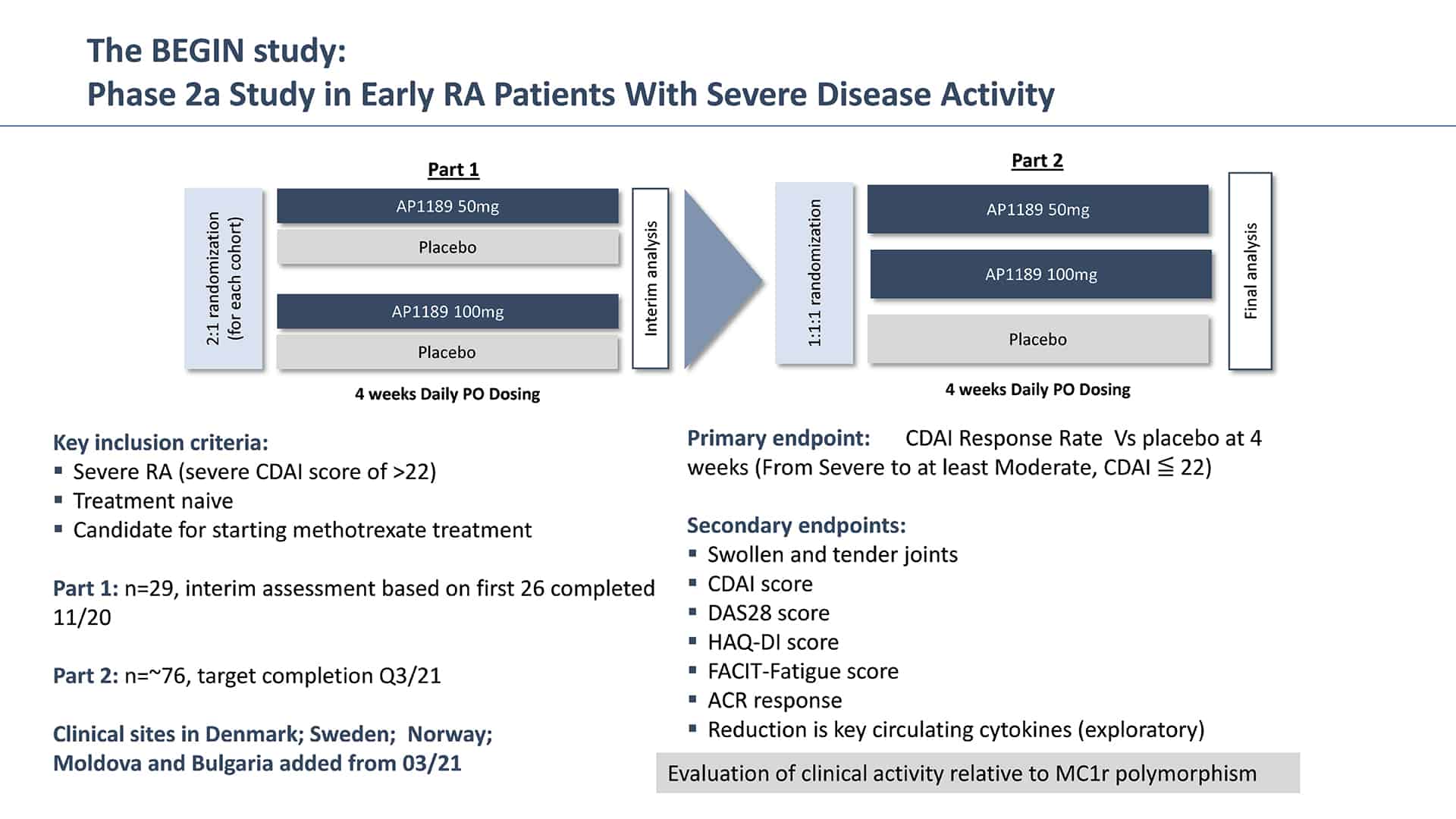
In November 2021, SynAct announced results from the Phase 2a study of resomelagon (AP1189) in newly diagnosed and previously untreated RA patients presenting with severe disease activity. The study, called BEGIN, was a randomized, double-blind, placebo controlled multicenter study in previous treatment naïve RA patients where either 50 mg or 100 mg of resomelagon (AP1189) or placebo was administered in addition to methotrexate (MTX). MTX is a disease modifying anti-rheumatic drug (DMARD) that is typically used as a first line of therapy. MTX tends to work well in most patients, but it can take up to 6-8 weeks for the drug to take full effect, and up to 40% of patients will not achieve a full response to MTX therapy and will require dose escalation or the addition of additional drugs like biological therapies which can induce a higher degree of immunosuppression.
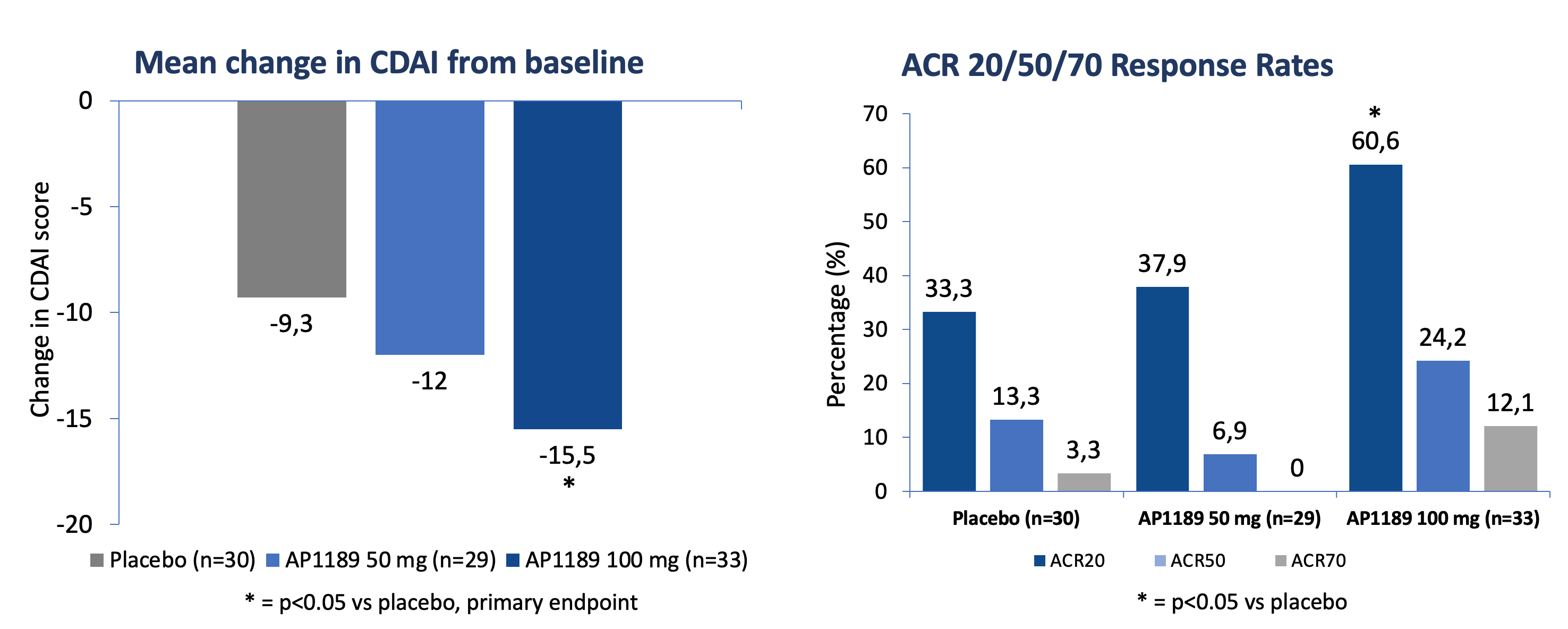
Resomelagon (AP1189) given once daily for four weeks was safe and well tolerated in the applied patient population. 100 mg of resomelagon (AP1189) demonstrated a statistically significant mean reduction in the clinical disease activity index (CDAI), the primary study endpoint, from baseline to four weeks that was more than 65% higher than the effect seen in the placebo-treated control group (mean reduction in CDAI: resomelagon (AP1189) 100 mg (n=33): 15.5 points compared with placebo (n=30): 9.3%, p = 0.0394). The 100 mg resomelagon (AP1189) group also demonstrated a significantly higher fraction of patients achieving ACR20 than placebo treated patients (ACR20: resomelagon (AP1189) (n=33) 100 mg: 60,6%; Placebo (n=30): 33.3%, P=0.0437) within the 4 weeks.
Based upon this initial promising data, SynAct announced a trial expansion in May 2021, increasing the overall trial size by up to 20 patients in order to increase the probability of demonstrating statistical significance in this POC trial. Trial recruitment is completed and SynAct expects to conclude the trial in Q4 of this year with top-line presented by the end of November, 2021.
Potential Utility of resomelagon (AP1189) in RA
The emerging clinical profile of resomelagon (AP1189) could potentially support multiple uses in the treatment of RA:
| Emerging resomelagon (AP1189) Clinical profile | |
|---|---|
| Oral Dosing | oral solid formulation to be used in the next clinical trial |
| Quick Onset of Action | as early as 2-weeks |
| High Response Rate | within 4 weeks |
|
Safe and Well Tolerated
No Immunosuppression
|
no treatment limiting Aes seen to date |
| Steroid-Free MoA | potential to be steroid sparing |
| Compatible with MTX | no known theoretical DMARD drug interactions |
Continued Development
Based upon the results of the BEGIN RA study, the company has conducted two additional Phase 2 clinical studies in RA with resomelagon (AP1189).
EXPAND – A 12-week Phase 2b study of daily resomelagon (AP1189) in MTX-naïve patients with severe disease activity
The EXPAND study was designed to test the treatment effect of 12-weeks of resomelagon (AP1189) on disease activity as measured by the ACR20 response rate as well as other RA disease measures and to confirm the safety profile of the molecule. The figure below provides an overview of the design of EXPAND.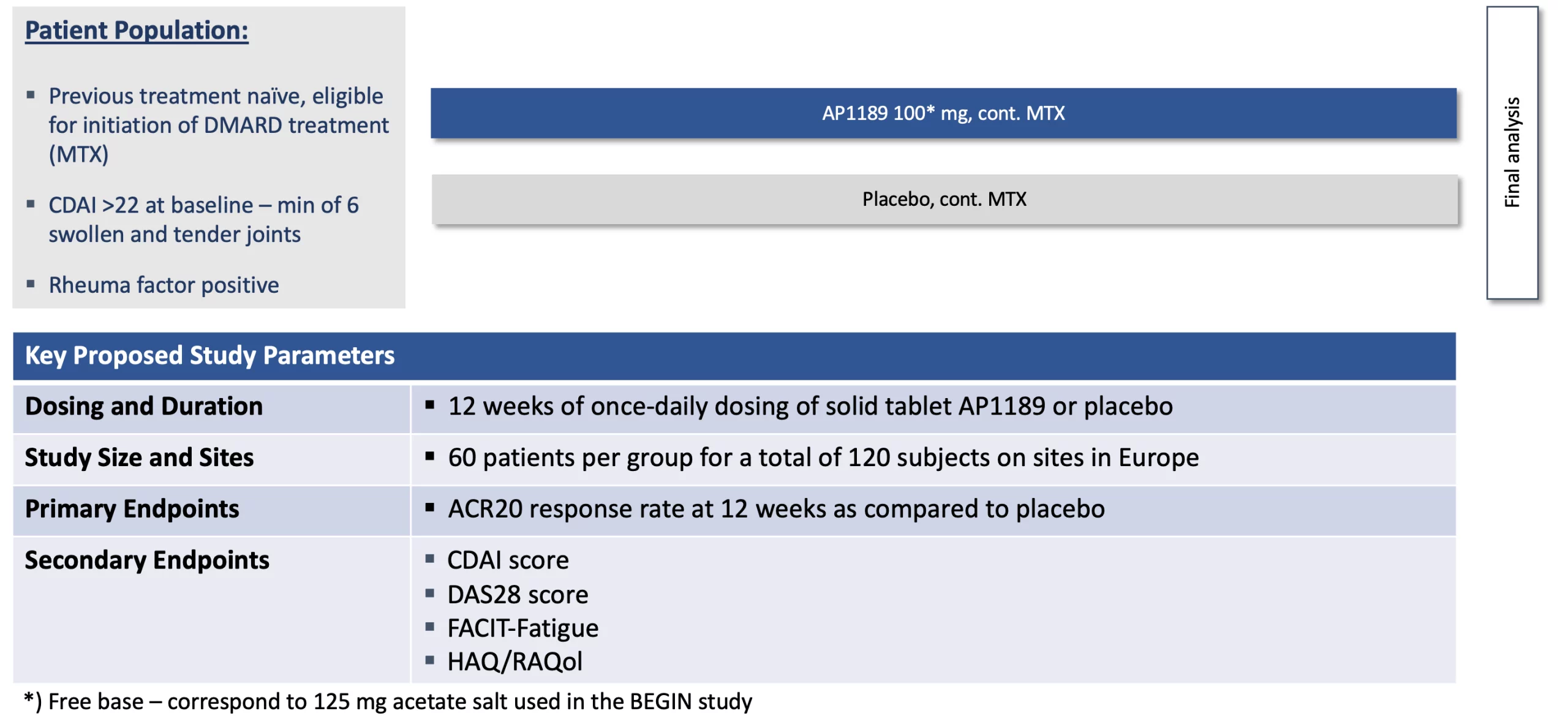
127 patients presenting with high disease activity (CDAI > 22) were randomized 1:1 for treatment with once daily 100 mg resomelagon or placebo added to a background of methotrexate (MTX) therapy. 54.7% of patients treated with 100mg of once-daily oral resomelagon achieved an ACR20 response at 12-weeks as compared to 55.7% of patients receiving placebo.
Resomelagon continued to demonstrate a favorable safety profile in this high activity patient population. The overall rate of treatment emergent serious adverse events (SAEs) was 1.6% (n=2), with 1 SAE in each group. The overall rate of patients experiencing treatment emergent adverse events (AEs) was 44.4% and 42.2% for resomelagon and placebo treated patients respectively (all patients received MTX). There were no observed signs of immunosuppression seen in the resomelagon group over that associated with background methotrexate therapy.
A post-hoc analysis was conducted of treatment naïve rheumatoid arthritis patients who had an elevated baseline level of C-reactive protein (CRP> 3mg/L), marker of systemic inflammation, and who were enrolled within 6 months of their diagnosis of rheumatoid arthritis (RA). In this subpopulation of patients, 100mg of daily resomelagon demonstrated a consistent and statistically significant response to therapy over placebo across assessed outcome measures.
At 12 weeks in EXPAND patients with elevated baseline CRP, the 100mg resomelagon group had an ACR20 attainment of 71% as compared to 54% of placebo patients. At 12 weeks in the subpopulation of patients with elevated CRP who were enrolled and had treatment initiated within 6mo of their RA diagnosis, 23 out of 28 (82%) of patients treated with 100mg resomelagon attained an ACR20 compared to 14 out of 27 (52%) of placebo patients (P<0.05).
A significant difference favoring resomelagon was also seen across the secondary outcome measures including the reduction in CDAI (resomelagon 24.6 vs placebo 14.7 points, p<0.01), reduction in DAS28-CRP (resomelagon 1.9 vs placebo 1.2 points, p<0.01), and reduction in HAQ disability index (0.69 vs placebo 0.31 points, p<0.05).
Safety in the elevated CRP population with treatment within 6 months of was comparable to what has been previously reported for the full EXPAND study population. [From PR Feb 22, 2024]
RESOLVE – 12-week Phase 2a/b study of daily resomelagon (AP1189) in patients with an incomplete response to first-line disease modifying anti-rheumatic drugs (DMARD-IR) who are experiencing moderate to severe disease activity.
A large percentage of patients treated with DMARDs never achieve the full desired effect, have a diminishing treatment effect, or suffer from side effects that can prevent further treatment. These patients who experience an inadequate response to DMARDs are referred to as DMARD-IR (inadequate responder).
The Company believes that resomelagon (AP1189) could be very well suited for DMARD-IR patients given the emerging profile of an efficacious, safe, and well tolerated once-daily oral therapy.
RESOLVE part A was conducted under a US Investigational New Drug application as a four arms, multi site, study with treatment of 60 mg, 80 mg, 100 mg resomelagon (AP1189) or placebo once daily for 4 weeks.
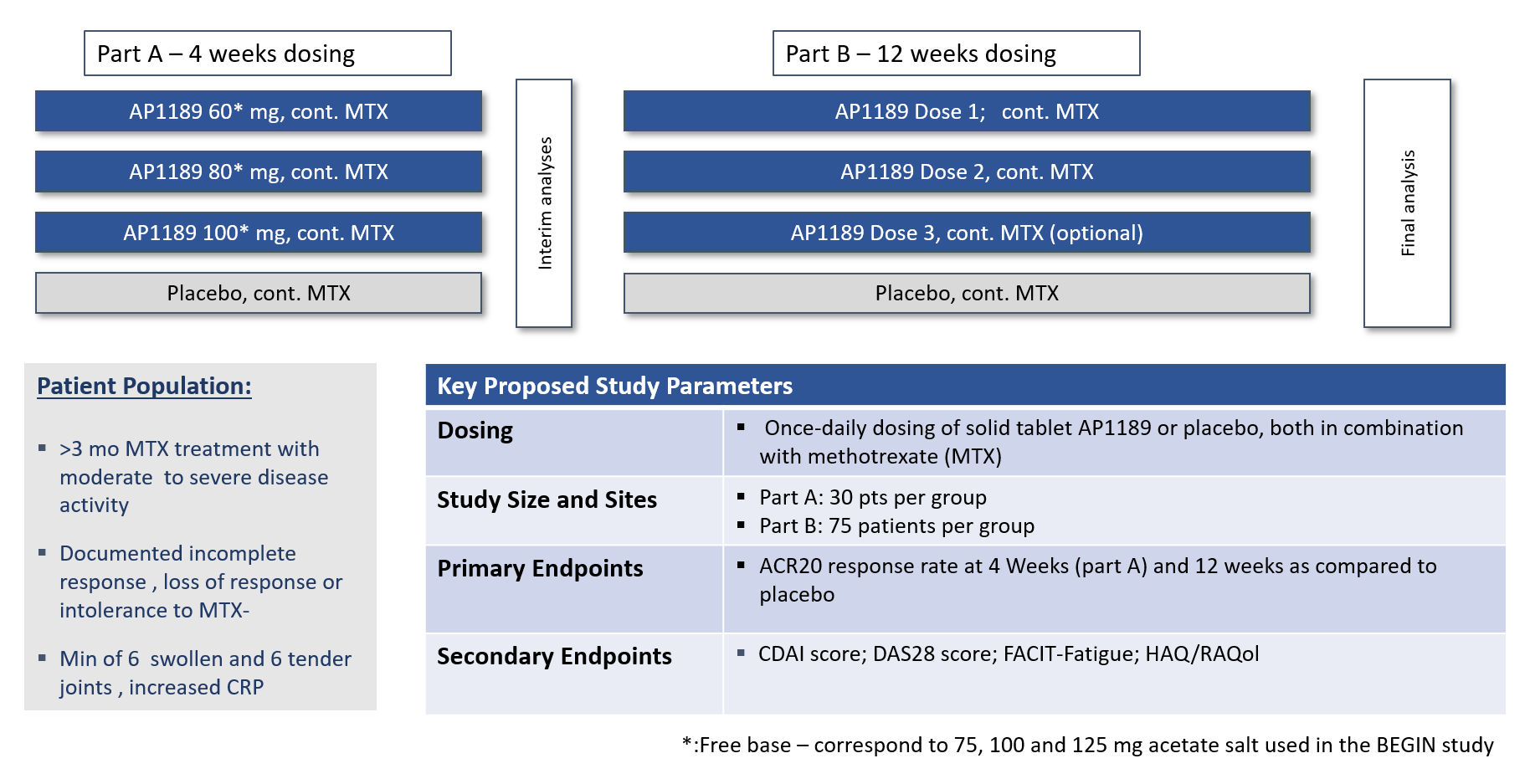 In total 125 patients were included of which 107 completed Resolve Part A per protocol with daily doses of placebo, 60mg, 80mg or 100 mg resomelagon for 4 weeks in addition to a stable dose of MTX.
In total 125 patients were included of which 107 completed Resolve Part A per protocol with daily doses of placebo, 60mg, 80mg or 100 mg resomelagon for 4 weeks in addition to a stable dose of MTX.
Efficacy data from one site has to be excluded from the efficacy analyses, SynAct is awaiting final efficacy assessments from the contract research organization (CRO) with the site removed from the calculations. However, the study showed a very high placebo effect with ACR20, the primary efficacy readout, around 50% at 1-month and with lower numbers reached in the three active groups.
Resomelagon continued to be generally safe and well tolerated relative to placebo. One serious adverse event (SAE) was reported in the study in a placebo treated patient that was hospitalized due to severe exacerbation in joint pain. The total number of treatment emergent adverse events (TEAEs) reported was 56 in the 125 recruited patients with 16 in the placebo group and 10, 12 and 18 in the 60mg, 80mg and 100mg resomelagon groups, respectively.
A large degree of heterogeneity in the recruited patient population was identified upon analysis of the study. Two-thirds of the patients had been on methotrexate (MTX) treatment for more than one year at the time of recruitment, with most patients being treated for over 2 years with only 5 completed patients having a medical history of initiation of MTX treatment within 6 months of RA diagnosis. In addition, medical history, as reported, did not support treatment with maximal tolerable dose of methotrexate in a fraction of the recruited patients
Further development in phase 2b will initially be focused on the patient population identified as responders to the resomelagon (AP1189) treatment in EXPAND. Detailed study design is being laid out in order to submit a clinical trial application and/or a protocol to the relevant health.
Development of resomelagon (AP1189) for the treatment of DMARD-IR patients remains an opportunity, but the patient population needs to be carefully selected in view of the compounds mode of action, and the benefit for the patients to be treated.