Our Lead Program: Resomelagon (AP1189)
SynAct’s drug candidate, resomelagon (AP1189), is a once-daily oral selective melanocortin agonist. Resomelagon (AP1189) selectively stimulates the melanocortin receptors that are directly involved in inflammation and its resolution without stimulating the adrenal glands to release cortisol. This selectivity enables resomelagon (AP1189) to exert its anti-inflammatory and immune resolution effects in a steroid-free manner without the significant safety, tolerability, and side effect issues associated with adrenocorticotropic hormone (ACTH) based therapies. Resomelagon (AP1189) is also a biased agonist that does not stimulate melanocortin pathways that are responsible for off-target activity like skin hyperpigmentation.
The company is evaluating resomelagon (AP1189) in three Phase 2 clinical programs: rheumatoid arthritis (RA), idiopathic membranous nephropathy (iMN), a form of nephrotic syndrome, and virus-induced respiratory insufficiency (VIRI) like that seen in COVID-19. In 2021, SynAct successfully completed Phase 2a trials in early severe RA and in hospitalized patients with COVID-19-induced respiratory insufficiency. Also in 2021, SynAct successfully tested a new oral solid tablet formulation of resomelagon (AP1189) in healthy volunteers and filed additional composition patents that should provide molecule exclusivity past 2040.
In 2023, the Company completed two new Phase 2 clinical trials in RA: EXPAND a Phase 2b trial in newly diagnosed RA patients experiencing severe disease activity and Part A of RESOLVE a Phase 2a/b trial in RA patients experiencing an incomplete or loss of response to methotrexate. In addition, the ongoing Phase 2a iMN trial was amended in 2022 to introduce the new oral tablet dosage form and to increase the treatment period to 3 months. The primary efficacy end points in EXPAND and RESOLVE were not met, but post hoc analyses indicate activity in better defined patient population and in view of the safety profile, these data support further development of resomelagon for the treatment of RA.
Phase 1 Development
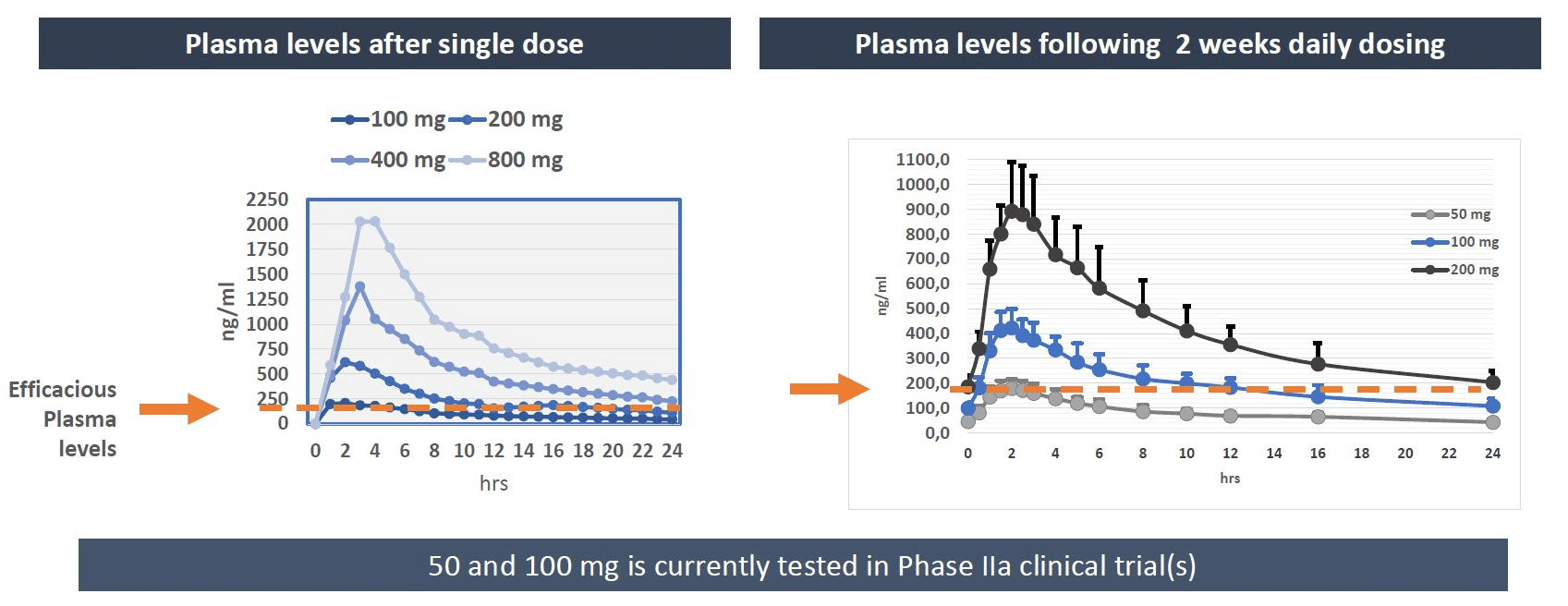
In the Phase 1 clinical assessment, 2 weeks of once-daily dosing of resomelagon (AP1189) supported continued development as a once-daily orally dosed medication. The plasma concentration needed induce pharmacological efficacy was reached within 1.5 hours of dosing and daily exposure is increased until steady state has been reached following 5-6 days of treatment after which no further drug accumulation was observed.
A comparative PK study in healthy volunteers of dosing with the suspension used in the early clinical phases and the tablet applied in EXPAND and RESOLVE, showed a similar PK profile for resomelagon (AP1189) after dosing either of the two dosage forms.