Disease Background
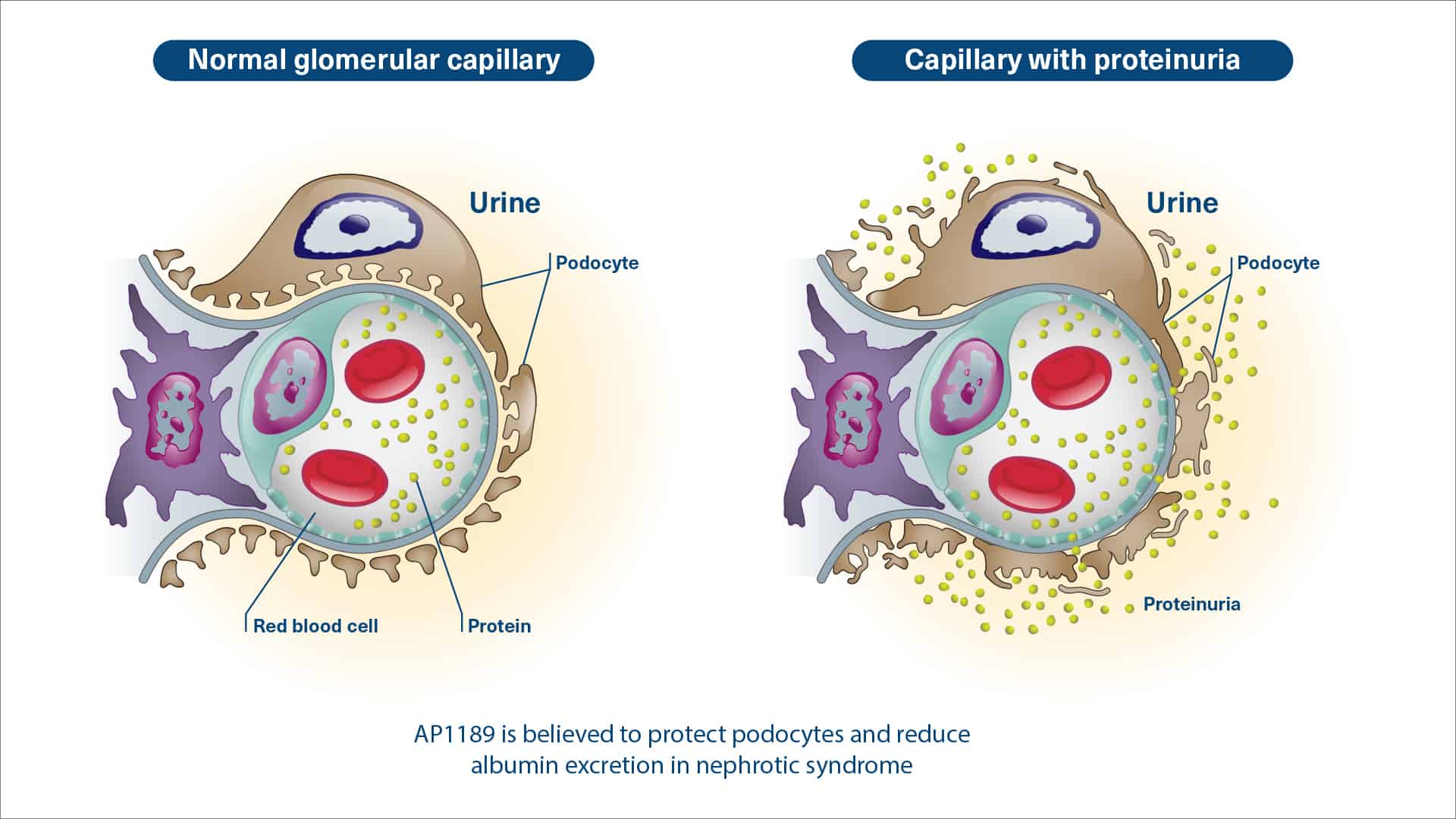
Nephrotic Syndrome (NS) is a condition associated with increased loss of protein into the urine resulting in tissue swelling and eventually development of edemas. The edemas can develop in the hands, feet, ankles, and face. Edemas can even develop in the lungs where it is associated with dyspnea (shortness of breath).
Untreated or insufficiently treated NS will in many cases be associated with hypercholesterolemia, increased risk for blood clots, increased risk for infections and can develop into chronic kidney disease that is associated with increased risk of development of cardiovascular disease and risk of development of end stage kidney disease and thereby need for renal replacement therapy (dialysis or transplant).
Membranous nephropathy (MN) is one of the frequent causes of NS. MN can be primary or it can be secondary to other diseases, including systemic lupus (lupus nephritis), cancer or seen following treatment with certain drugs.
Clinical development of resomelagon (AP1189) in idiopathic membranous nephropathy (iMN)
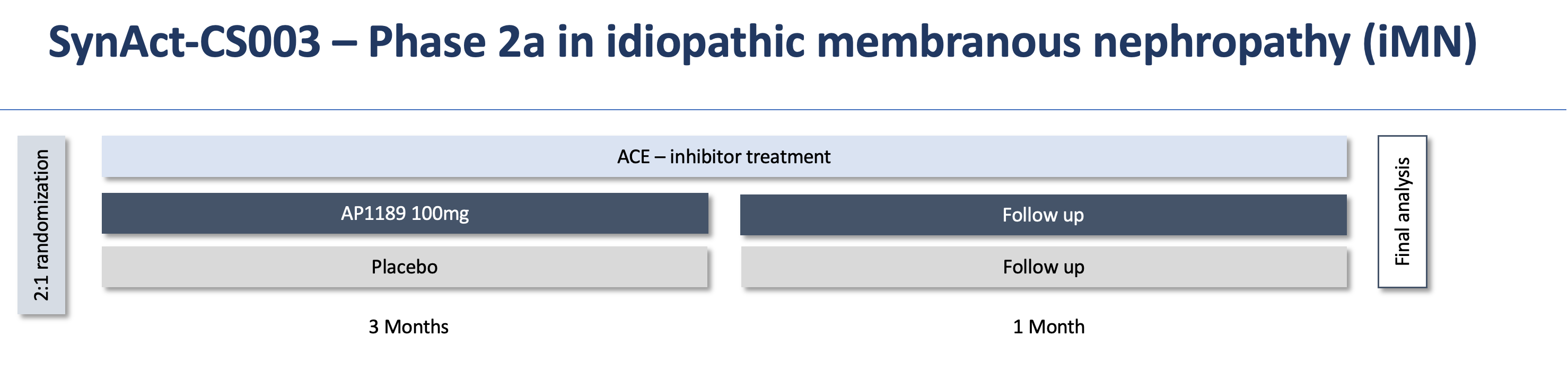
Resomelagon (AP1189) is being tested in an exploratory, randomized, double-blind, multicenter, placebo-controlled Phase 2a study with repeated once-daily 100 mg dosing to assess the safety, tolerability, pharmacokinetics, and efficacy of resomelagon (AP1189). The study population consists of patients with iMN with severe proteinuria who are on an ACE inhibitor or angiotensin II receptor blocker treatment.
In July 2022, the company submitted a protocol amendment which was approved in September. This amendment introduces the new oral tablet as well as a longer 3-month dosing duration. The benefit of this redesign is that it increases the likelihood to show significant treatment effect on urinary protein excretion, the main efficacy read-out in the study, and increase patient compliance as a once-daily dosing with a tablet is much more convenient than daily intake of an oral suspension. The figure
below provides an overview of the design of the iMN clinical trial.
- The Phase 2a study in iMN was paused in Q4-21 in order to amend the study protocol to a 3-months treatment period and to utilize the new oral solid tablet.
- Protocol amendments were filed in July 2022 and the study was resumed in H2 2022.