Rheumatoid arthritis (RA) is a global disease. Currently approximately 18 million people worldwide live with RA, and about a million new patients are diagnosed each year. The numbers are increasing, most prevalently in industrial countries, driven by factors such as population growth, age expectancy, lifestyle, and improved health care.
What is RA
RA is an autoimmune/chronic inflammatory disorder that typically affects more than just the joints. RA is an autoimmune disorder, a disease where the immune system mistakenly attacks your body’s own tissues. RA affects the lining of the joints, causing a painful swelling that can result in cartilage and bone erosion and joint deformity.
Which symptoms are associated with RA
RA is often associated with symptoms involving other parts of the body including skin, eyes, lungs, heart, and blood vessels. Initial symptoms are in most cases joint pain, arthralgia, but without the cardinal arthritis symptoms of tender and swollen joints. Symptoms of arthritis gradually develop over time. Sometimes over weeks in other cases over months to years.
Today’s Treatment Options
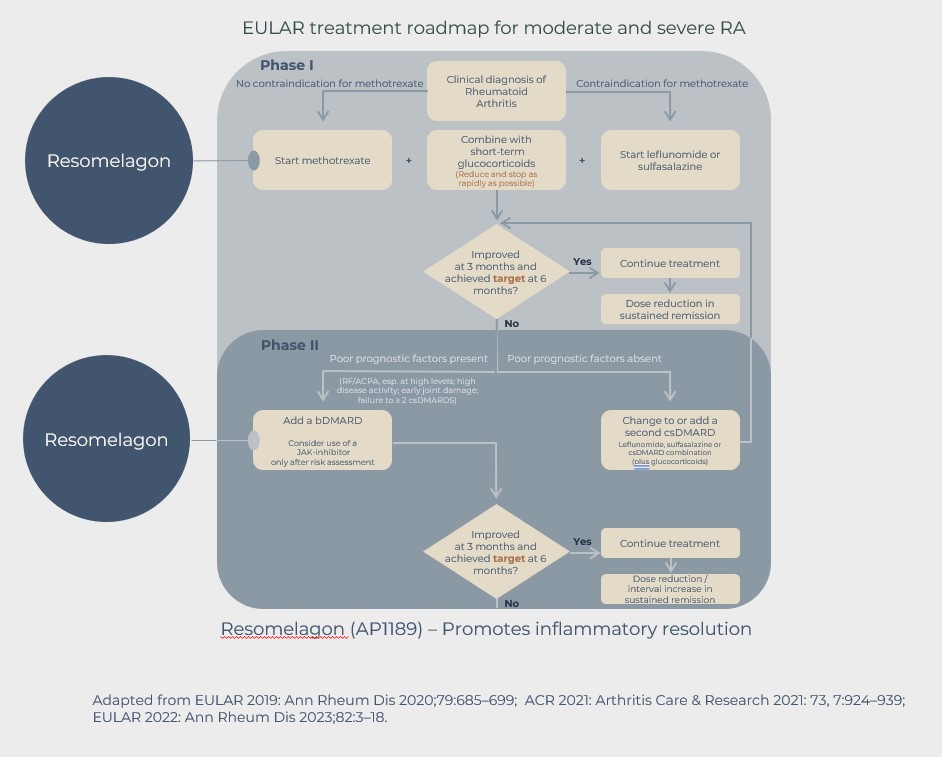
According to guidelines (EULAR and FDA in Europe and US respectively) the treatment of these patients is not initiated before the diagnosis of RA. The diagnosis can, due to lack of access to a rheumatologist, be significantly delayed and often patients, while waiting to be evaluated by a rheumatologist, are insufficiently treated with painkillers including NSAID (non-steroid anti-inflammatory drugs) and even with glucocorticoids.
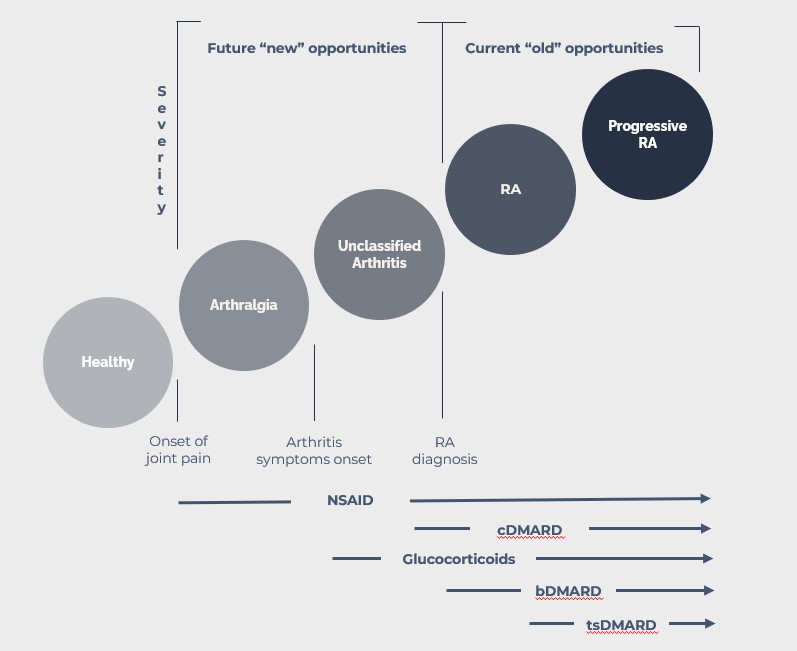
Following the RA diagnosis, patients with moderate to high disease activity will as first line treatment in most cases be treated with the conventional disease modulating anti-rheumatic drug (cDMARD) methotrexate (MTX). MTX has a slow onset of action and a reduction in symptoms cannot be expected in the first weeks of treatment.
The MTX treatment is therefore often combined with dosing of glucocorticoids, resulting in very fast reduction of symptoms, but glucocorticoids are associated with unwanted side-effects. The guidelines state that the use should be restricted to short term, and the dose of glucocorticoids should be tapered as fast as possible and be redrawn completely within 3 months. However, many RA patients are treated chronically with glucocorticoids.
The goal for first line treatment is to get disease control within 3- 6 months from start of treatment. Disease control is defined as low disease activity or disease remission. Many patients are responding inadequately to the first line treatment and will not experience disease control in the expected time period.
In such cases an alternative treatment, such as a biological DMARD, e.g. a TNF-inhibitor, which can induce a higher degree of immunosuppression, can be added to or can be replacing the MTX treatment. Some patients will not respond well to this treatment either or stop responding over time, in which case a third treatment option, such as an alternative bDMARD or the so-called targeted synthetic DMARDs, such as the JAK inhibitors, should be offered to the RA patients.
The Treatment Opportunity
While new types of medications have improved treatment options, significant unmet needs still exist. For patients not in disease control, RA still progresses, and damage accumulates. Patients often cycle through therapies and classes of therapies and must deal with periods of acute disease activity called flares, which can occur several times per year, and drive the need to adjust the dose of current drugs or to change to a new therapy to maintain control of the disease.
The treatment of glucocorticoids is often prolonged for an extended period of time, some data indicates that up to 50% of all RA patients will have prolonged or even chronic use of GCs thereby increasing the risk of severe side effects.
Ill controlled RA causes more patient interaction with the health care system and reduces the well-being of the patient.
A patient-friendly and safe treatment option as an add on to first line treatment, that would induce disease control in a glucocorticoid sparing fashion, with the further potential to reduce and/or delay the introduction of second line treatments, might fulfill some of the unmet needs of the RA patients.
The development of resomelagon offers such an opportunity, and if successful the treatment may fit well into the treatment guidelines for RA and fulfill some of the unmet needs.
