Disease background
Virus infected patients can develop a variety of (severe) symptoms due to (hyper)inflammation and the immune system being out of control.
Virus Induced Respiratory Insufficiency
Virus infections affecting the lungs – such as COVID-19 and influenza – can for some patients lead to severe respiratory insufficiency, which can be life threatening. These patients has difficulty breathing and therefore they are unable to provide enough oxygen to vital organs. The patients are hospitalized and receives oxygen supplementation to survive. The patients often develop severe pneumonia, which makes the situation even more complicated. If not treated, the patient may develop into acute respiratory distress syndrome (ARDS), a very serious condition where the patient requires mechanical ventilation in order to have enough oxygen for the organs.
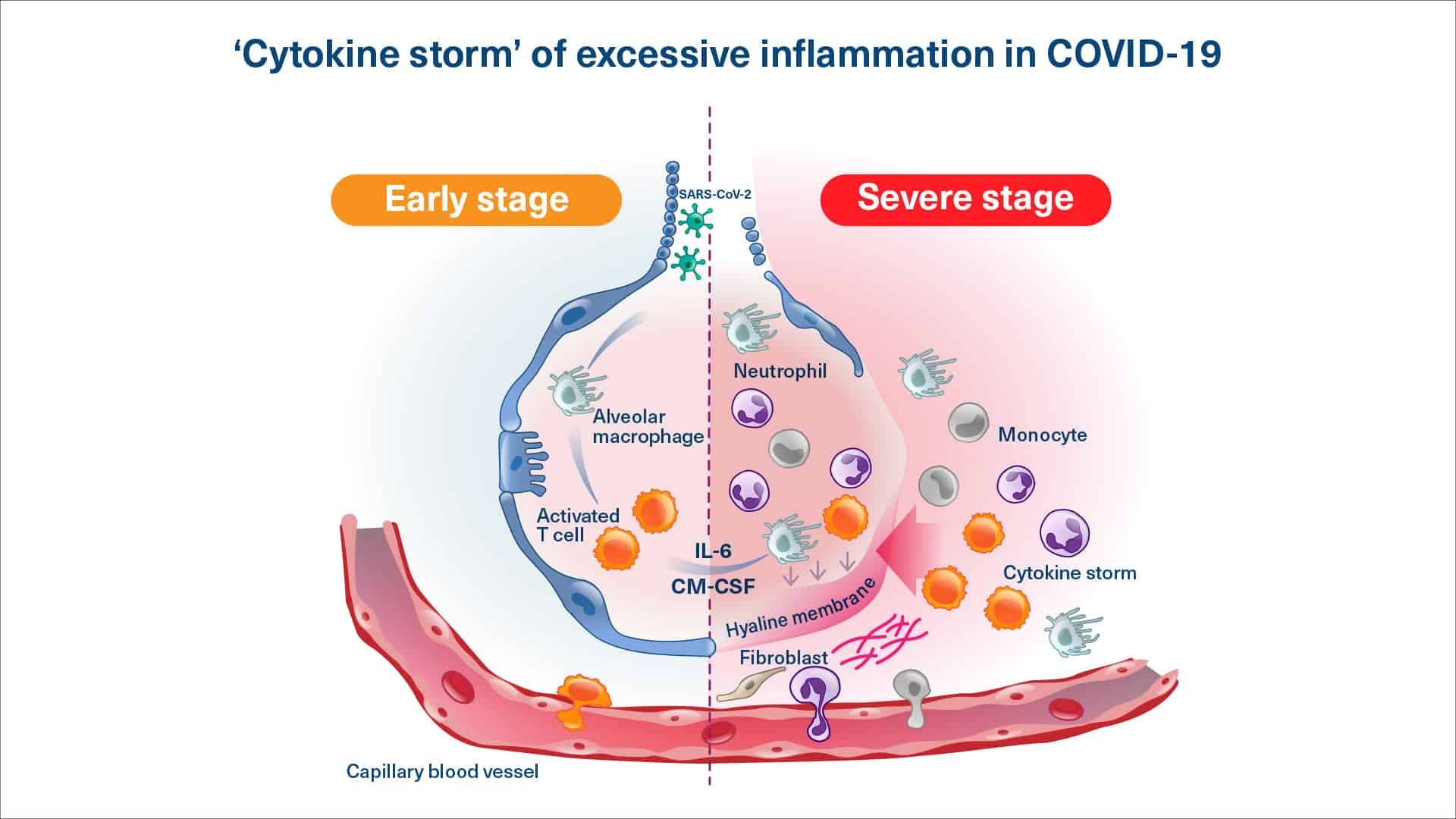
Viral or secondary bacterial infections can cause the immune system to be overly active and produce excessive quantities of pro-inflammatory molecules (a ‘cytokine storm’, also known as Systemic Inflammatory Distress Syndrome or SIDS) which in itself can cause damage to key organs.
Virus induced organ damage due to hyperinflammation
Several viral infections can result in a cytokine storm and hyperinflammation, where the immune system is out of balance. As a consequence, key organs may be affected. Such a harmful response of the immune system is e.g. seen in some patients infected dengue virus.
Severe dengue fever can cause internal bleeding and organ damage. The blood pressure can drop to dangerous levels, causing shock – and in some cases, severe dengue fever can lead to death. Dengue virus are spread to people by bites of infected mosquitoes and ticks.
Dengue virus have now also spread to Europe and US – probably due to temperature changes. Viruses like Chikungunya and Zika virus are also increasing.
RESOVIR Collaboration
To investigate the potential for resomelagon (AP1189) in treatment of virus infections, we formed the RESOVIR (Resolution Therapy for Viral Inflammation Research) collaboration. RESOVIR is a scientific and clinical collaboration between Professor Mauro Teixeira, MD, PhD, Universidade Federal de Minas, Belo Horizonte, Brazil, Professor Mauro Perretti, PhD William Heavy Research Institute, Barts and the London School of Medicine, Queen Mary University, London, UK, and SynAct Pharma.
The RESOVIR collaboration focus on the potential of resomelagon as an add-on to current treatment in viral disorders, where the immune system has reacted with excessive inflammation and being out of balance.
Preclinical in vitro, ex vivo, and in vivo models, have demonstrated effect of resomelagon (AP1189). This includes animal models of SIDS, where resomelagon have been shown to selectively reduce inflammation and stimulate macrophages to clean-up the inflamed tissue. Models of viral infection and clinical studies are also performed as a part of the RESOVIR collaboration.
Clinical development of resomelagon (AP1189) in virus-induced hyperinflammation
In the first study, an investigator-initiated trial, RESOVIR-1 hospitalized COVID-19 patients were treated with 100 mg AP1189 as an add-on treatment to the other treatments received. In the second investigator-initiated trial RESOVIR-2, patients with severe dengue fever will receive resomelagon treatment on top of conventional symptomatic treatment.
The two proof-of-concept investigator-initiated trials will support that resomelagon can be used as an add-on treatment in patients with virus induced inflammation and the immune system being out of balance.
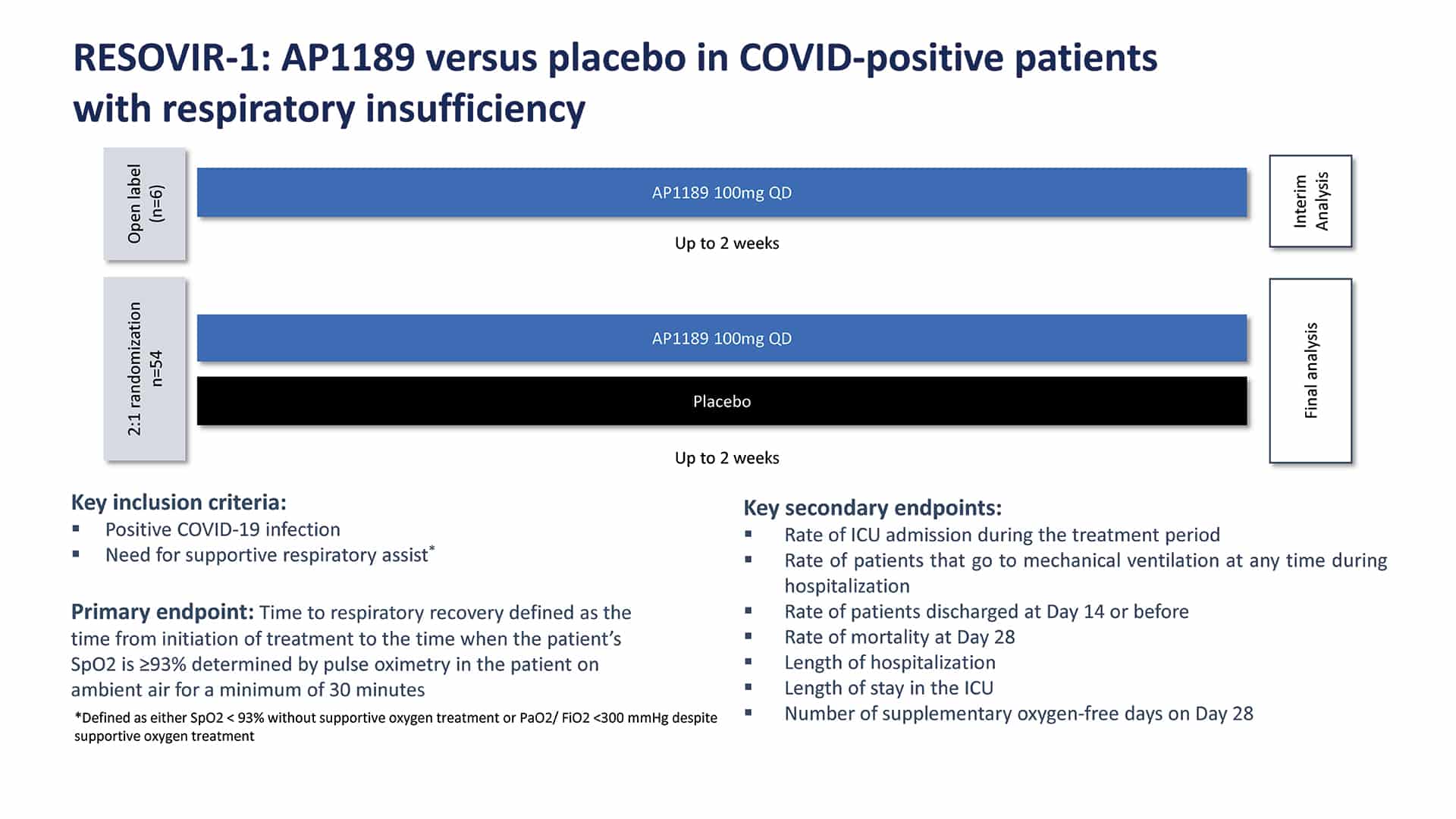
RESOVIR-1: Resomelagon (AP1189) versus placebo in COVID-positive patients with respiratory insufficiency
We designed and executed a 60 patient Phase 2a clinical trial in Brazil. 60 hospitalized COVID-19 patients who required supplemental oxygen (experiencing respiratory insufficiency) were enrolled in this investigator-initiated trial in Brazil. . All patients received steroids (dexamethasone) at an average dose of 6mg/day. After an initial open-label safety run-in of 6 patients, the blinded placebo-controlled portion of the trial dosed an additional 36 patients with 100mg of resomelagon (AP1189) and 18 patients with placebo, each given orally once-daily for 2 weeks.
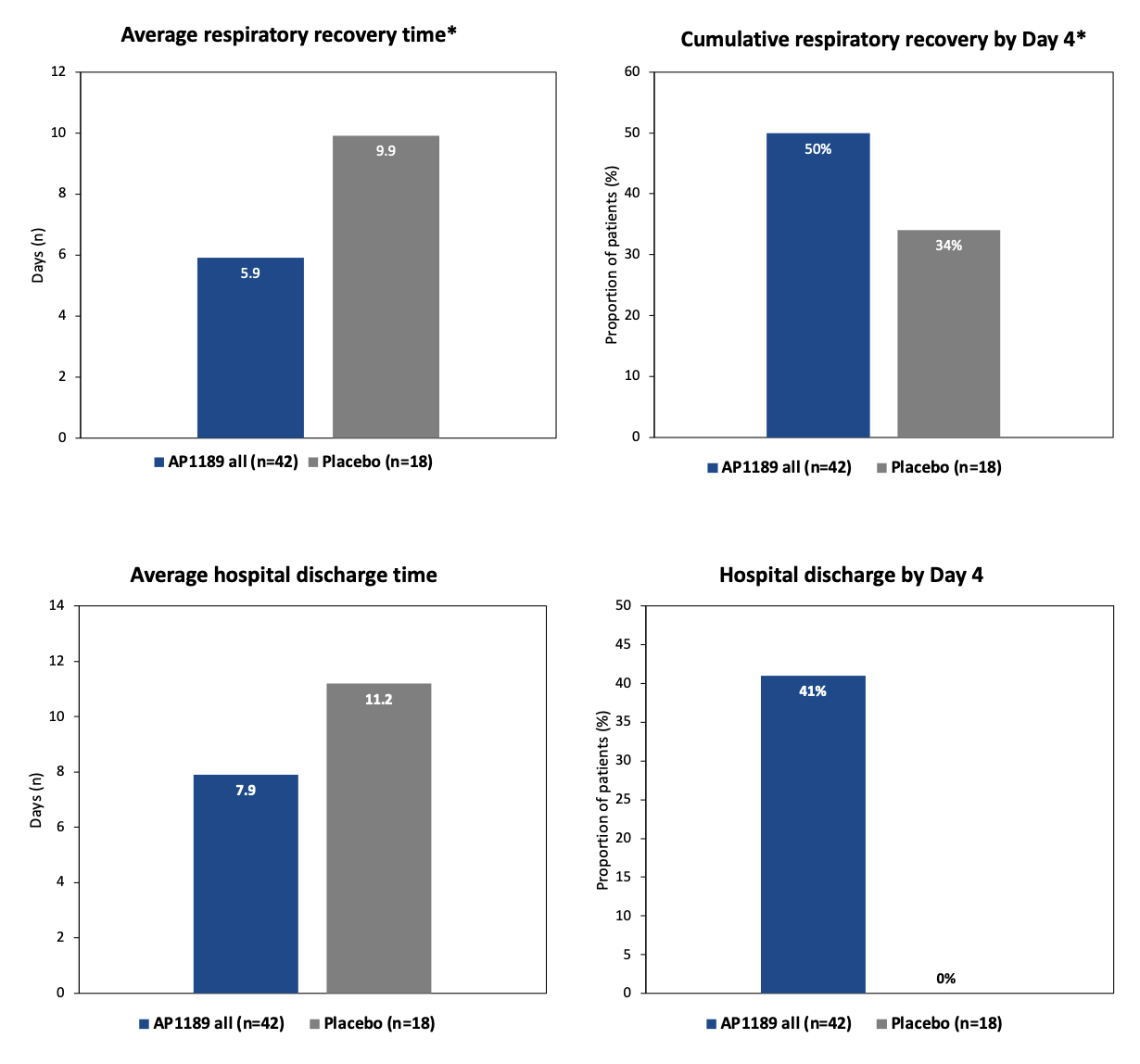
Patients treated with 100mg resomelagon (AP1189) orally once-daily for 2 weeks achieved respiratory recovery (no longer requiring oxygen therapy) on average 3.5 days (35%) quicker than placebo treated patients (6.4 days and 9.9 days on average respectively). All resomelagon (AP1189) treated patients (including the first 6 open-label safety patients) recovered respiratory recovery on average 4.0 days (40%) quicker than placebo treated patients (5.9 days and 9.9 days on average respectively). Resomelagon (AP1189) patients were discharged on average 3.3 days earlier than placebo and by day 4, 41% of resomelagon (AP1189) patients had been discharged vs 0% for placebo.
RESOVIR-2: Treatment of Patients with Dengue
The clinical proof-of-concept as an add-on treatment to conventional symptomatic treatment in virus-induced inflammation, will also be done in patients infected with dengue virus.
The RESOVIR-2study is a randomized placebo-controlled, phase II study (investigator-initiated) testing once daily oral dose of Resomelagon (AP1189) vs placebo (1:1 randomization, n=120) as add on to standard treatment in patients with symptomatic dengue.
The potential effect will be evaluated by time to disease resolution by a composite clinical end point. Other clinical end points including the ability to reduce the incidence of warning signs of and/or the development of severe dengue will also be evaluated.
The study is ongoing in Brazil with Professor Mauro Teixeira, MD, PhD as principal investigator.